Development futureproofs hospital’s drug preparation capabilities
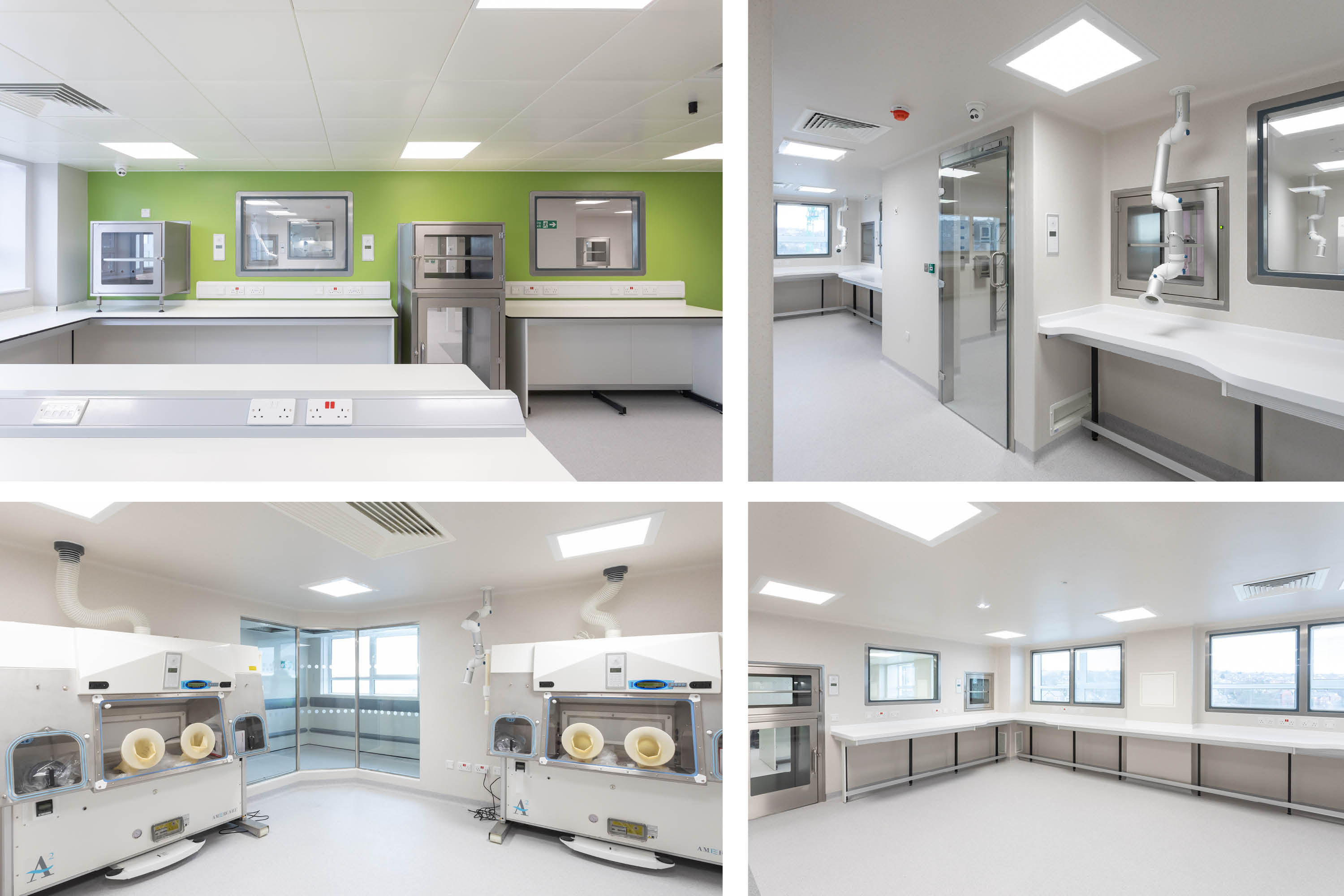
BES has completed a £2.5m project to create a new pharmacy and aseptic suite at Weston Park Hospital for Sheffield Teaching Hospitals NHS Foundation Trust.
Designed, engineered, and constructed by BES, the new facilities aim to futureproof the hospital’s drug preparation capabilities onsite, with an ATMP-classified environment that enables the production of regenerative medicine, personalised treatments, and the development of nanomedicines.
Working collaboratively with the client in the role of both principal designer and principal contractor, BES designed the project for delivery in two phases, enabling the hospital’s existing pharmacy to continue operating on a business-as-usual basis until the new pharmacy was ready for use.
To create the new facilities, BES refurbished a redundant office area on the sixth floor of the hospital to create the new, high-specification chemotherapy aseptic dispensing facility in phase 1.
Following commissioning and validation of this facility, the hospital’s pharmacy team moved across from the former pharmacy, vacating that area to allow the phase two refurbishment of the former pharmacy as the new aseptic suite with sophisticated clinical trial (CT) and ATMP preparation facilities.
Working with the hospital’s user requirement specification (URS), and engaging with the hospital’s estates team and senior pharmacist, BES’s multidisciplinary team took the project concept design from RIBA stage two to stage 4, delivering buildability and value engineering benefits.
And the company’s integrated design and construction capability meant the need to maintain business-as-usual conditions and uninterrupted pharmacy services during the programme could be embedded in the design process, resulting in smoother project delivery.
The facility supports the hospital’s reputation for excellence in cancer treatment and creates a template for future gene therapy preparation facilities for other NHS trusts
BES designed the new facilities to HTM and cGMP pharmaceutical requirements, carrying out the design qualification validation process before work began on site.
And design development also involved consultation with the Medicines and Healthcare Regulatory Authority (MHRA) to ensure the design of the facility is compliant with regulatory requirements and to ensure a smooth validation process.
This resulted in further design improvements to the aseptic suite design to avoid any cross-contamination risk.
Delivering the project in BIM, BES designed the facilities using 3D REVIT modelling, along with Navisworks for clash detection.
Lumion software was also used to transfer the model into rendered images to give the client a realistic view of what the facility would look like, and virtual reality walk-throughs allowed staff to experience the facility as part of a collaborative design development process.
Jonathan Morton, the BES engineering director responsible for leading the project, said: “This project leveraged our multi-disciplinary approach to co-ordination of architectural design, building services engineering, and construction, along with our collaborative approach to working with the client and end-user stakeholders.
“With responsibility for maintaining business as usual within the live hospital environment, and for health and safety as principal designer and principal contractor, we considered the needs of clinicians, service users, and patients as an integral part of the design and delivery process.
“The result is a facility that supports the hospital’s reputation for excellence in cancer treatment and creates a template for future gene therapy preparation facilities for other NHS trusts.”